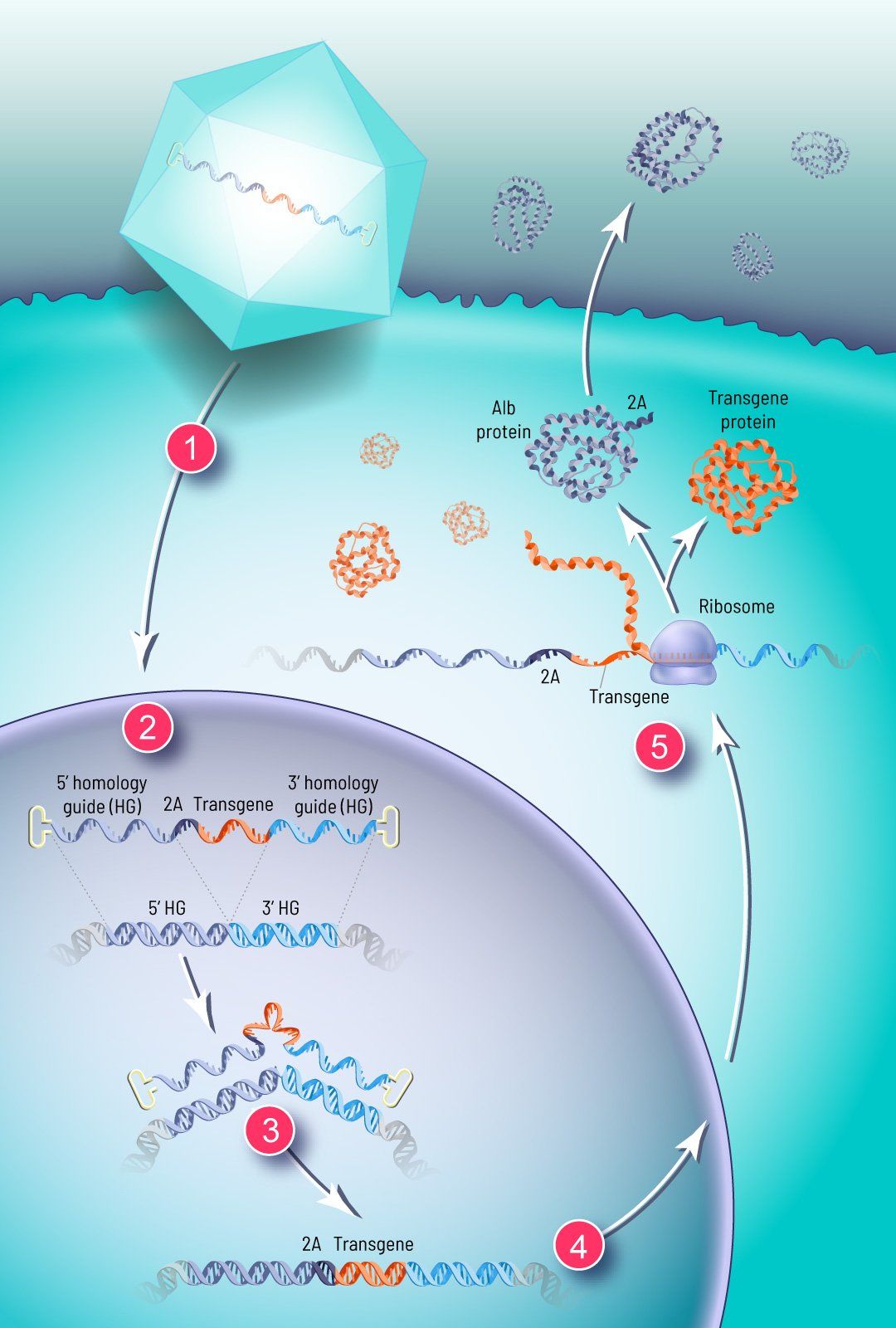
How GeneRide works
We use a synthetic viral vector to deliver the corrective transgene to the nuclei of the patient’s cells via an infusion
(1).
Two “homology guides” — strands of DNA several hundred base pairs long that precisely match a specific stretch of the patient’s own genome — flank the corrective transgene
(2).
Upon sensing the therapeutic DNA in the nucleus, the cell’s natural DNA repair machinery responds and integrates the corrective transgene at a specific site in the patient’s genome (3). The transgene is inserted in the same place every time, in the chromosome and at the gene that corresponds to the DNA sequence encoded in the homology guides.
For our liver-targeted therapies, this specific location for integration is within the albumin locus. Albumin is the most abundant protein in circulation and the most highly expressed gene in the liver. This high expression level is driven by the albumin promoter, which is very strong and tissue specific. We have demonstrated
integration at other target loci and expect to develop GeneRide for other target tissues like CNS and muscle in the future.
When our therapeutic transgene is integrated in the albumin locus, downstream of the albumin coding region, it can hitch a ride on this endogenous promoter to drive expression of the corrective transgene the patient has been lacking, without disrupting albumin production
(4). By using an element called a
2A
peptide, we are able to efficiently produce albumin and the transgene as two separate proteins and modify albumin with a small tag that allows us to monitor GeneRide activity in a non-invasive manner
(5).
Shortly after treatment, the modified cells begin producing the therapeutic protein to combat the disease.
The GeneRide advantage
GeneRide is our proprietary platform for precise and durable genome editing. We believe it has the potential to provide safety and efficacy benefits over existing technologies.
The challenge
In traditional gene therapy, the corrective genes do not integrate with the patient’s chromosomes but remain floating inside the nucleus. This means that the corrective gene is not carried through to successive generations when cells divide. The therapeutic effect is thus diluted over time — especially in children, whose cells divide rapidly as they grow, and in tissues such as the liver, where cells also divide and regenerate in adults.
Durability
The GeneRide difference
Our GeneRide platform harnesses the cell’s natural DNA repair process to integrate the corrective gene into the patient’s chromosome at a precise and predetermined spot. The gene then persists as an integral part of the patient’s DNA as cells divide, with the potential to lead to a more durable therapeutic effect.
The challenge
Traditional gene editing technologies use engineered nucleases, such as CRISPR/Cas9, TALEN and zinc fingers, to cut the patient’s DNA and remove or insert a target gene. This process is not precise; it has been shown to lead to off-target cuts and uncontrolled deletions or insertions in the patient’s chromosomes. These changes to the patient’s DNA can raise the risk of genomic instability and other genotoxicities. The use of nucleases, which are typically derived from bacteria, can also raise the risk of provoking strong responses from the body’s immune system.
Precision
The GeneRide difference
Our platform does not require exogenous (foreign) nucleases. Instead, it leverages the body’s natural DNA repair process to integrate the corrective gene at a specific location every time. This reduces the risk of off-target and potentially dangerous changes to the patient’s DNA. Because we do not introduce exogenous nucleases, we also reduce the risk of immune reaction.
The challenge
Traditional gene therapy approaches rely on an exogenous “promoter” to drive expression of the therapeutic transgene. The transgene is not designed to integrate into the patient’s chromosome, but a low level of random integration does occur. This is not precise, and there is a documented risk that the promoter could land next to and change the expression of other genes in the patient’s chromosome, including oncogenes. This raises the risk of cancer and other genotoxicities.
Safety
The GeneRide difference
Our platform does not use exogenous promoters, but rather harnesses existing promoters in very specific locations in the patient’s chromosomes. That reduces the risk of cancer as compared to traditional gene therapy approaches. Studies in multiple animal models demonstrate the potential safety and efficacy of the GeneRide platform (see publications for more information).
©2023 LOGICBIO THERAPEUTICS, INC. Privacy Policy | Terms of Use